Once Upon a Time in the Green Valley
Fosun Pharma is paying 1.4 billion yuan for a drugmaker with a notorious past
Fosun Pharmaceutical (600196.SH, 02196.HK) is acquiring a controlling stake in Green Valley Technology, the developer of sodium oligomannate (GV-971), betting it can revive a drug long marketed as China’s first homegrown treatment for Alzheimer’s disease.
Skepticism has followed GV-971 from the start. Chinese and international experts have questioned whether it can meaningfully slow—let alone reverse—Alzheimer’s-related cognitive decline. Seeking global validation, Green Valley launched an international Phase III study in 2020 targeting roughly 2,000 patients across China, the U.S., and Europe, but enrollment fell well short and the trial was terminated in 2022. On the regulatory front, the drug’s prospects also darkened in 2025, when its Chinese license lapsed and renewal remained under review.
Yet even if GV-971’s final verdict is still pending, Green Valley’s earlier record in China is not. Long before Alzheimer’s, there is a sustained pattern of touting purported anti-cancer products with sweeping claims and repeat advertising violations—rebranding products and shifting between “health product” and “drug” identities to keep sales going.
For many Chinese families, that history is not an abstract compliance story but a remembered calamity. Desperate cancer patients and their relatives were drawn in by sheer lies for products that did not work. They spent heavily—and worse, they spent their last precious time clinging to false hope, squandering what may have been the final chance to pursue evidence-based care. When hope finally collapsed, many were left facing a brutal, irreversible end.
Legally, Fosun is buying a defined corporate stake in a specific entity; reputationally, many Chinese people see continuity in the leadership and brand lineage. And to this very day, Green Valley traces its founding to 1997, effectively opening itself to inquiries to its checkered past.
Recent English-language coverage has largely focused on the Alzheimer’s drug’s evidence base, the conditional-approval process, and the economics of restarting trials—while skating past the earlier “miracle cure” era that Chinese reporting had already put on the record.
So here is part of that record: a 2008 report by China Central Television, the state broadcaster.
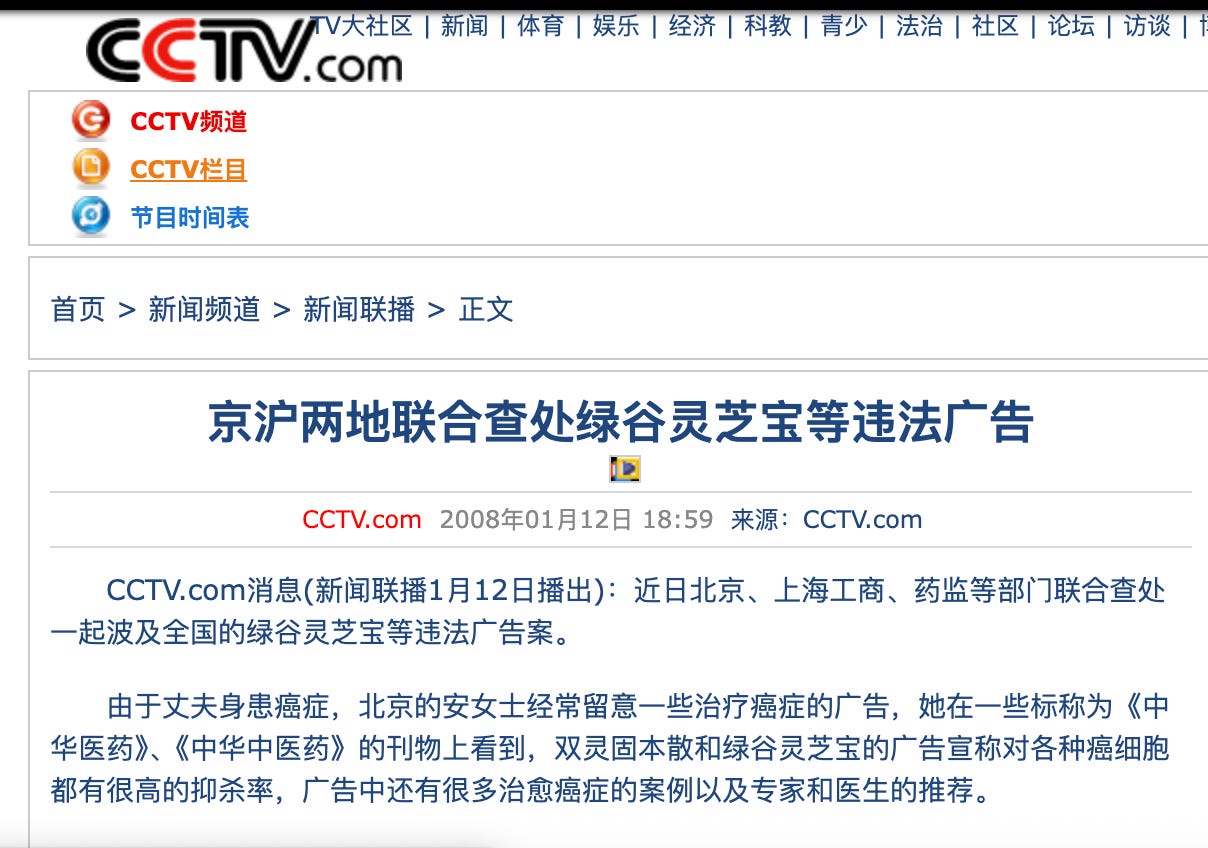
京沪两地联合查处绿谷灵芝宝等违法广告
Beijing and Shanghai jointly crack down on illegal advertisements for products such as Green Valley Lingzhi Bao
by state broadcaster China Central Television (CCTV) on January 12, 2008
Aired on Xinwen Lianbo/News Simulcast on January 12, 2008
Recently, the industry and commerce administrations, drug supervision authorities, and other relevant departments in Beijing and Shanghai jointly investigated and dealt with an illegal advertising case involving products such as Green Valley Lingzhi Bao, a case whose impact extended nationwide.
Because her husband suffers from cancer, Ms. An in Beijing often pays close attention to advertisements claiming to treat cancer. In some periodicals labeled Zhonghua Yiyao (“Chinese Medicine”) and Zhonghua Zhongyiyao (“Chinese Traditional Chinese Medicine”), she saw advertisements for Shuangling Guben San and Green Valley Lingzhi Bao claiming to have a high inhibitory and killing rate against various cancer cells. The advertisements also included many cases claiming cures for cancer, as well as recommendations from “experts” and doctors.
Ms. An: “There were medical PhDs, and recommendations from some experts and scholars, so I felt it couldn’t be fake.”
However, after spending nearly 20,000 yuan on Shuangling Guben San and Green Valley Lingzhi Bao, Ms. An’s husband’s condition did not improve at all. In interviews, China Central Television reporters found that in recent years, in places such as Beijing, Shanghai, and Guangzhou, many family members of patients who had taken these products reported to relevant authorities that the advertisements for the two products were suspected of false promotion. This drew close attention from industry and commerce administrations, drug supervision authorities, and other departments in Beijing, Shanghai, and elsewhere.
After investigating and collecting evidence, law enforcement authorities found that starting in 1996, the Shanghai Green Valley Group successively launched three “generations” of so-called anti-cancer products named Zhonghua Lingzhi Bao, Shuangling Guben San, and Green Valley Lingzhi Bao. The false advertisements for these products all claimed clear anti-cancer effects, and cited a large number of scientific research institutions, authoritative hospitals, and patients, among others, to conduct false publicity about their products’ efficacy—misleading a large number of patients over a period as long as ten years.
Under China’s relevant regulations, drug advertisements must not contain content that uses the names or images of patients, experts, or doctors as endorsements; and advertisements for health foods must not directly or indirectly promote therapeutic effects.
Tang Minhao, Deputy Director of the Shanghai Food and Drug Administration: “There is subjective intent. You clearly know the law has explicit provisions, and I have already told you, warned you, and even penalized you, yet you still continue to commit the offense. In such circumstances, this should be considered a serious illegal act.”
At present, the case is still being further handled.
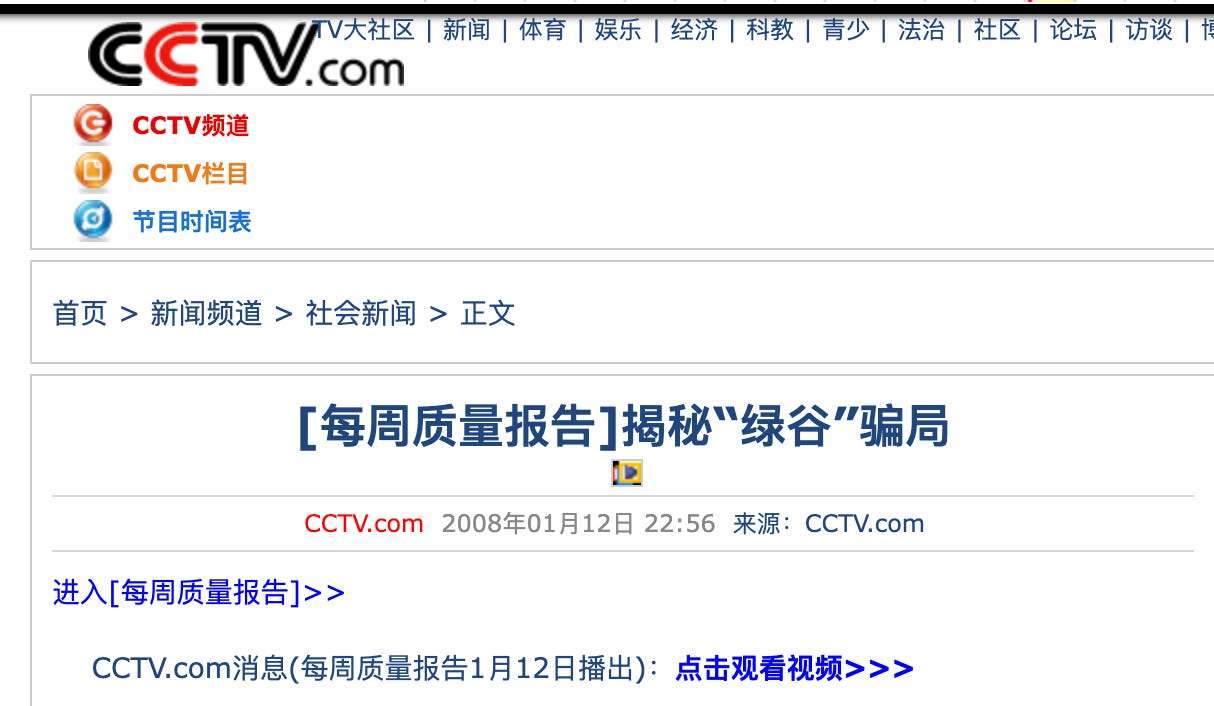
[每周质量报告]揭秘“绿谷”骗局
Weekly Quality Report: Exposing the “Green Valley” Scam
by state broadcaster China Central Television (CCTV) on January 12, 2008
Aired on Meizhou Zhiliang Baogao/Weekly Quality Report on January 12, 2008
Host: Working together to build a high-quality life—this is Weekly Quality Report. Hello, everyone. “False drug advertisements are fiercer than tigers.” Some may say that sounds a bit exaggerated, but the reality is this: false advertisements for medicines and health products that wildly exaggerate efficacy and mislead patients keep appearing one after another. They have become a “chronic ailment” threatening consumers’ medication safety, a major public hazard and social scourge. Recently, Shanghai’s drug regulatory authorities exposed a batch of illegal advertisements. One of the companies involved kept changing its name and promotional claims, allowing false advertising to run rampant for more than a decade, misleading large numbers of cancer patients and causing extremely negative social impact—becoming one of the most typical series of false-advertising cases nationwide in the past ten years.
Recently, the Shanghai Food and Drug Administration issued its November 2007 public notice on illegal advertisements for medicines, medical devices, and health foods, exposing illegal ads related to 17 products. Among them, a product claiming to be a new-generation anti-cancer product—Green Valley Lingzhi Bao—previously publicized and exposed multiple times by enforcement authorities in various localities, once again appeared on the blacklist. The reason for this exposure was still the same: it released product advertisements without approval and engaged in false promotion.
Tang Minhao, Deputy Director, Shanghai Food and Drug Administration: They clearly know the law has very explicit provisions, and we have already told them, warned them, and even disciplined them, yet they continue to offend. Green Valley’s conduct, I would say, seriously violates national regulations on advertising—drug advertising—or other relevant provisions. In such circumstances, this should be regarded as a serious illegal act.
China Central Television reporters note that not long before this, in a monitoring operation by the Beijing Food and Drug Administration targeting illegal advertisements for medicines and health foods, Green Valley Lingzhi Bao was likewise placed on the blacklist for releasing unapproved advertisements without authorization.
Shanghai FDA enforcement officers told the reporter that since 2004, repeated monitoring has found that the Green Valley Group has released illegal and false advertisements, and that the forms of the illegal ads are varied.
Meng Qiying, Inspection Detachment, Shanghai Food and Drug Administration: It publicizes that the inhibition-and-killing rate against a leukemia cell line is 94.6%—94.6% is almost 100%. Just imagine: as a patient, you’re bound to feel you want to give it a try. Everyone tries it; most leukemia patients, after seeing this kind of false promotion and being misled, will try it—then their product gets sold, doesn’t it?
According to China’s Interim Provisions on the Examination of Health Food Advertisements, advertisements for health foods must not directly or indirectly promote therapeutic effects, nor may they explicitly or implicitly suggest that the health food has disease-treatment effects by touting the functions of certain ingredients.
However, in Green Valley Lingzhi Bao’s illegal advertisements, the reporters observed that the company claimed Green Valley Lingzhi Bao has four major anti-cancer effects: directly inhibiting tumors; increasing clinical cure rates; increasing tumor surgical resection rates; and preventing tumor metastasis and recurrence.
According to Shanghai’s drug regulatory authorities, beginning in 1996, the Shanghai Green Valley Group successively launched three “generations” of so-called anti-cancer products: Zhonghua Lingzhi Bao, Shuangling Guben San, and Green Valley Lingzhi Bao. The false advertising for all three generations followed the same pattern, each claiming outstanding anti-cancer effects. In July 2001, China established a public-notice system for illegal drug advertisements. From that point on, Zhonghua Lingzhi Bao became a frequent “regular” on the blacklist in those notices. And because it repeatedly released illegal and false advertisements, in December 2002 it was prohibited from placing advertisements in mass media.
Yet right at that time, Zhonghua Lingzhi Bao began its first “change of face,” renaming itself Shuangling Guben San, and obtaining a national drug approval number (“Guoyao Zhunzi”). Using its status as a drug, it launched a new round of false advertising. According to incomplete statistics, since 2001, Shuangling Guben San has appeared nearly 800 times in illegal-advertisement notices issued by drug regulators across the country.
In April 2007, Shuangling Guben San’s drug approval number was revoked by the State Food and Drug Administration because of falsified application materials. After that, the Shanghai Green Valley Group changed its appearance again and rolled out what it called its third-generation anti-cancer product—Green Valley Lingzhi Bao—making a second “change of face” and continuing to heavily promote the product’s purported miraculous effects in treating all kinds of cancers.
Drug regulators verified that in the advertising for Green Valley’s series of anti-cancer products, the company cited large numbers of scientific research institutions, authoritative hospitals, experts and scholars, and “benefited” patients to promote the supposed anti-cancer effects of its three successive generations of products. It wove for the products a halo of claims such as: “the world’s first Ganoderma (lingzhi)-based anti-cancer drug,” “China’s number one anti-cancer traditional Chinese medicine,” “the only anti-cancer traditional Chinese medicine selected for the ‘United Nations Procurement Catalogue,’” and “the first Chinese new drug to enter U.S. FDA clinical certification.” It was precisely through these tempting promotions that large numbers of cancer patients were drawn to purchase Zhonghua Lingzhi Bao, Shuangling Guben San, and Green Valley Lingzhi Bao, treating these products as their lifeline.
At present, the Shanghai FDA has transferred advertisements suspected of serious violations by the Green Valley Group to Shanghai’s industrial and commercial authorities for case filing and investigation.
In September 2006, the husband of Ms. An in Beijing was unfortunately diagnosed with esophageal cancer. Just as the whole family was nearly in despair, advertisements for Shuangling Guben San and Green Valley Lingzhi Bao made the family see hope again. Although China’s Standards for the Examination and Release of Drug Advertisements stipulate that drug advertisements must not publicize cure rates or effective rates, Green Valley Group’s advertisements nevertheless claimed that its products specifically treat various late-stage cancers, have good inhibitory-and-killing rates against all kinds of cancer cells, with a 93.6% inhibitory-and-killing rate against a human liver cell line, and an inhibitory-and-killing rate reaching 100% against human leukemia cell lines and lung cancer cell lines.
In Shuangling Guben San’s advertisements, Ms. An also saw a large number of so-called authoritative experts giving full affirmation of the drug’s efficacy.
Ms. An: There were professors and experts from American universities, medical PhDs and the like, as well as medical PhDs from the Chinese Academy of Sciences, and presidents of traditional Chinese medicine (TCM) hospitals from other places, veteran TCM doctors—people like that, all seemed legitimate. After reading it, you feel that if they all say it’s good and recommend the medicine—how good it is—then it can’t be fake. If ordinary people eat it, you might not believe it and think it’s fake. But so many senior experts said it, so you believed it.
However, under China’s Standards for the Examination and Release of Drug Advertisements, drug advertisements must not contain content that uses the name or image of experts or doctors as endorsements.
Ms. An told the reporter that what ultimately moved her and her husband most was the large number of examples listed in the advertisements. In those cases, many cancer patients were in worse condition than Ms. An’s husband, yet the advertisements said that after taking Shuangling Guben San, their cancers had already been cured and their bodies restored to health.
Ms. An: The key is that there were so many examples that attract people—some had lung cancer, some liver cancer, this cancer, that cancer. So many people took it and got better—after so long (they got better). Some didn’t even have surgery; they just used their products. Anyway, they all had names. There was a woman in her 30s with breast cancer; after surgery she didn’t do chemo or radiotherapy, she just kept using this product—so it wouldn’t harm your white blood cells. Now, tests are all normal. Just taking the medicine and using the medicine—maybe half a year—then it was normal, and the cancer cells hadn’t spread.
China’s Standards for the Examination and Release of Drug Advertisements also stipulate that drug advertisements must not contain content that uses patients’ names or images as endorsements. Yet both Shuangling Guben San and Green Valley Lingzhi Bao advertisements included numerous case stories to “prove” their cancer-curing efficacy. According to the ads, these patients suffered from many different cancers, but they all shared one thing in common: after taking Green Valley Group’s series of products, they had recovered. The temptation of high cure rates, expert and fellow-patient recommendations, plus the fact that these ads were published in seemingly professional newspapers with names like Zhonghua Yiyao (“Chinese Medicine”) and Zhonghua Zhongyiyao (“Chinese Traditional Chinese Medicine”)—all of this made Ms. An feel even more assured.
Ms. An: These newspapers looked like (they were) legitimate newspapers—something like the Chinese Medical Association and so on, and then some business papers—looked pretty legitimate. It can control the development of cancer cells and can extend your life.
But after spending nearly 20,000 yuan and taking Shuangling Guben San and Green Valley Lingzhi Bao for more than half a year, Ms. An’s husband’s condition did not improve noticeably as the advertisements claimed.
Reporter: After your husband took this medicine, did he achieve the effect advertised in those (newspaper) case stories?
Ms. An: No. After he finished taking it, he didn’t feel it got better. After he took it, he started not wanting to eat much—nausea, vomiting. After vomiting, even after increasing the dosage, it just got worse and worse.
Ms. An’s husband was not as fortunate as the patients described in the advertisements. His condition continued to deteriorate. In August 2007, Ms. An’s husband passed away.
During the investigation, China Central Television reporters found that in recent years, in places such as Beijing, Shanghai, and Guangzhou, many family members of patients who had taken Zhonghua Lingzhi Bao, Shuangling Guben San, and Green Valley Lingzhi Bao filed complaints and reports with drug regulators, industry and commerce authorities, and other departments, saying these products did not have the various miraculous effects advertised. Quite a number of patients, like Ms. An’s husband, purchased and took Shuangling Guben San and Green Valley Lingzhi Bao, and still passed away.
At the Chaoyang Branch of the Beijing Administration for Industry and Commerce, enforcement officers told the reporter they had investigated the newspapers that once persuaded Ms. An—Zhonghua Zhongyiyao (“Chinese Medicine”), Zhonghua Zhongyiyao (“Chinese Traditional Chinese Medicine”), Zhonghua Zhongyiyao Bao (“Chinese Traditional Chinese Medicine Newspaper”), and the like.
Liu Lijun, Deputy Chief, Advertising Division, Chaoyang Branch, Beijing AIC: The names of these two supposed news organizations are quite similar to “Zhonghua Yiyao.” There are two real news organizations. One is 中国中医药报社, and the other is 中华中医药杂志社. Those two names are the closest matches, so we asked those two real news organizations.
Reporter: And what were the results of your inquiries?
Liu Lijun: They said that this printed item “Zhonghua Yiyao” was not published by their news organization and had nothing to do with them.
At the Chaoyang AIC branch, the reporter saw certifications issued by those two real news organizations and magazine publishers: the so-called newspapers that carried the advertisements—Zhonghua Yiyao (“Chinese Medicine”), Zhonghua Zhongyiyao (“Chinese Traditional Chinese Medicine”), Zhonghua Zhongyiyao Bao (“Chinese Traditional Chinese Medicine Newspaper”), and so on—had no relationship whatsoever with China Traditional Chinese Medicine News or Zhonghua Zhongyiyao Magazine. So were these “newspapers,” which moved countless cancer patients, actually newspapers?
Liu Lijun: We went to the Beijing Municipal Administration of Press and Publication to ask whether this was a legally issued printed publication. The Press and Publication Administration said it was a printed item, not a periodical or magazine. First, it had no publication serial number—no serial number approved by the Press and Publication Administration. Second, it had no advertising business license, because for media—paper media, television media, and so on—to publish advertisements, they must register with the AIC and obtain an advertising business license before they can publish ads.
After verification by the Chaoyang District AIC, the so-called newspaper advertisements that carried Shuangling Guben San and Green Valley Lingzhi Bao ads were not nationally approved newspapers for distribution at all; they were merely printed advertising materials produced by the company itself. Enforcement officers told the reporter that such printed advertising materials are also illegal.
Liu Lijun: First, it’s a prescription drug. Prescription drugs must be advertised in professional media. At the same time, our Drug Advertisement Examination Standards clearly stipulate that prescription drugs cannot be advertised in the form of “giving away” medical or pharmaceutical periodicals.
Regarding the illegal advertising content for Zhonghua Lingzhi Bao, Shuangling Guben San, and Green Valley Lingzhi Bao, Shanghai’s drug regulators also conducted long-term investigation and evidence collection.
Shuangling Guben San advertisements claimed that it had passed a U.S. FDA application for a new-drug clinical trial and had been approved to enter U.S. clinical use as an anti-cancer drug. However, Shanghai’s drug regulators found during their investigation that the supporting materials provided by Green Valley Group differed greatly from the content promoted in the advertisements.
Zhang Shaohui, Inspection Detachment, Shanghai Food and Drug Administration: In 2004, they submitted an application concerning Shuangling Guben San for alleviating vomiting symptoms in chemotherapy patients. But the U.S. FDA’s reply to that application was that it confirmed receipt, and that within 30 days they could not begin clinical trials until the U.S. FDA further confirmed the completeness of the treatment information. Later, based on our investigation, Green Valley Group did not begin the clinical research work for that item. In 2005, they submitted another application, which did receive U.S. FDA approval, but that application was mainly a tolerability and safety test of an oral Ganoderma extract in a population of healthy volunteers. Up to now, none of the materials they provided can prove there was an approval allowing Ganoderma to enter U.S. clinical trials as an anti-tumor drug.
The advertising claim of “entering the U.S. as a clinical anti-cancer drug” was false. Similarly, Green Valley’s advertisements also claimed that its products were exported to 23 countries and regions around the world—including the United States, Europe, Japan, and Singapore—and that more than one million people had used them. Shanghai’s drug regulators further verified in their investigation that the so-called supporting materials provided by Green Valley Group for the United States, Japan, and Singapore likewise could not prove that its products were used overseas as anti-cancer drugs.
Fan Zhihong, Inspection Detachment, Shanghai Food and Drug Administration: After we intervened and began investigating, Green Valley only provided three pieces of proof. The first was proof of exporting to the United States—just a simple export customs declaration. After we looked into it, the quantity was only 1.92 kilograms—very little. On the other hand, for exports to Japan, they provided a document showing that as a food—Ganoderma—it had a microbiological test report; they provided no materials at all about how its sales were.
And in the third document, issued by Singapore’s health authority, although Shuangling Guben San was permitted to be exported to Singapore as a CPM (Chinese proprietary medicine), the Singapore health authority’s official website clearly states that CPM-type medicines are not allowed to promote effects such as treating cancer.
Fan Zhihong: They have no evidence whatsoever that in Japan or Singapore it is sold as a medicine, or as an anti-cancer medicine. They have absolutely no such evidence.
Regarding these advertising claims, Li Keliang, Deputy General Manager of Shanghai Green Valley Pharmaceutical Technology Co., Ltd., also acknowledged in a recorded interview with Shanghai’s drug regulators that content in the advertisements—such as Shuangling Guben San and Green Valley Lingzhi Bao being exported to 23 countries and regions worldwide and carrying out large-scale clinical experiments in the United States—was indeed exaggerated.
Drug regulators and industry-and-commerce enforcement officers pointed out that from Zhonghua Lingzhi Bao starting in 1996, to Shuangling Guben San after 2002, and to today’s Green Valley Lingzhi Bao, the Shanghai Green Valley Group has continuously changed names and promotional content, enabling false advertising to run rampant for more than a decade, misleading large numbers of cancer patients and causing extremely negative social impact—becoming one of the most typical series of false-advertising cases nationwide in the past ten years.
At the same time, this case also exposed certain loopholes in China’s legal and regulatory framework for drug advertising supervision.
Shan Baojie, Division Director, Department of Drug Market Regulation, State Food and Drug Administration: Generally speaking, people paid close attention to the Green Valley issue before the newly revised measures for drug-advertisement examination. Our entire drug regulatory system also stepped up monitoring of this product’s illegal advertising. But under the laws and regulations at that time, drug regulators could only make the monitoring results known to the public in the form of public notices.



China brings quacks to court. In the US they become health officials.